BrainTypeCreatineKinase
Brain-type Creatine Kinase (PDB ID:3DRB) from Homo
sapiens
Created by: Andrew Paisley
Human brain-type creatine kinase (CKB, 3DRB) is a
transferase protein belonging to the phosphagen kinase superfamily. Like all
phosphagen kinases, creatine kinase catalyzes a high-energy reaction involving
a phosphoryl group transfer. In this case, the phosphoryl group of an ATP is
transferred to creatine in a reversible reaction that forms ADP and
phosphocreatine (1). (Image reference no. 5)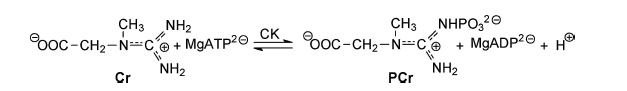 Phosphocreatine is stored in cells and later used as a
rapid source of high-energy phosphate in the form of ATP. The role of CK is
vital to cellular activity. The system functions as an ATP buffer, maintaining
an optimal ATP concentration for cellular activity (2).
Phosphocreatine is stored in cells and later used as a
rapid source of high-energy phosphate in the form of ATP. The role of CK is
vital to cellular activity. The system functions as an ATP buffer, maintaining
an optimal ATP concentration for cellular activity (2).
Creatine kinases in Homo sapiens enable the efficient
supply of ATP to skeletal muscle, the heart, and the brain. ATP is utilized
from the PCr pathway for biological processes that require a high level of
energy, generally in a rapid time span (1). Muscle-type creatine kinases are
functionally significant for rapid and powerful muscle contraction (3).
Creatine has gained recognition in the public eye as a supplement for explosive
resistance training. As such, muscle-type creatine kinase has been thoroughly
researched from an exercise physiology perspective. Brain-type creatine kinase
exists in the brain and the retina, and it functions to supply energy for ion
transport pumps in the brain (1). The brain uses the energy provided from the
PCr system to activate Na+-K+ ATPase ion pumps and ATP-gated
K+ channels (1). Such ion transporters are imperative for neural
cells, as they manage the membrane potential and propagate action potentials.
In total, these ion transporters account for 50% of the brain’s energy usage
(1). This role suggests that CKB is an important mechanism for proper brain
function.
Brain-type creatine kinase exists in the cytosol of
neural cells, and it forms as a homodimer (BB-CK) (1). The structure of this
globular protein has two domains: an N-terminal helical domain (residues 1-100)
and a C-terminal a/b
(residues 124-381) (1). Residues 101-124 link the two domains (1). Each monomer
has 381 residues, and a molecular weight of 42644.2 (4). The secondary structure of the monomer is 38% helical (19 helices) and 16% beta sheet (18 strands).
In its homodimer form BB-CK has a molecular weight of 85270.5. The theoretical
isoelectric point of BB-CK is 5.34 (4).
Creatine kinases catalyze the production of
phosphocreatine and ADP from creatine and ATP. To make this happen, CKB forms
multi-ligand structures during its reaction mechanism. The protein forms a
transition state analog complex (TSAC) consisting of ADP-Mg2+, NO3-,
and creatine (1). (Image reference no. 1)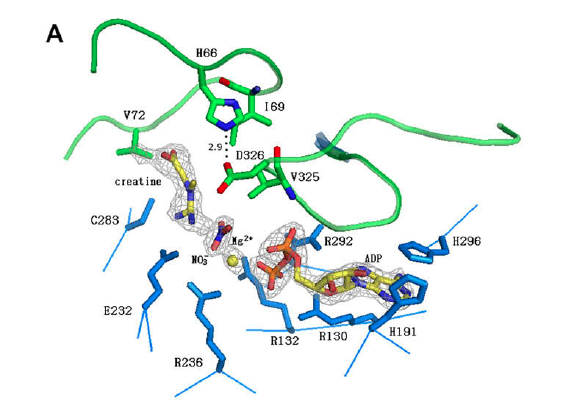 After a phosphoryl transfer to creatine, a phosphocreatine
molecule is released and an ADP-Mg2+ complex remains. Even though
CKB is a homodimer, these complexes exist in only one of its two monomers. The
active monomer is in the closed conformation. The other monomer exists in a
ligand-free, open conformation throughout the reaction (1). This being the case,
CKB is actually an asymmetric homodimer.
After a phosphoryl transfer to creatine, a phosphocreatine
molecule is released and an ADP-Mg2+ complex remains. Even though
CKB is a homodimer, these complexes exist in only one of its two monomers. The
active monomer is in the closed conformation. The other monomer exists in a
ligand-free, open conformation throughout the reaction (1). This being the case,
CKB is actually an asymmetric homodimer.
Research has uncovered a number of key amino acid
residues, which play a role in the catalytic activity of this protein. The
active site of CKB must accommodate all of the different ligand components. ADP fits into a binding pocket. The
adenine molecule of ADP is held in place by a stacking interaction with His296 (1). The nucleotide is further supported by hydrogen bonding between His191,
the 2’-hydroxyl of the ribose sugar, and the N from Gly294 (1). While research
has not yet uncovered the specific mechanism involving these residues, it is
known that His296 plays a vital role. If His296 is replaced by a different
amino acid, such as asparagine, there is a large decrease in substrate binding
affinity and catalysis (1,5). In order to stabilize the highly reactive
phosphate molecule, the phosphate binding pocket must be composed of multiple
positive charges. These positive charges come from five arginine residues: Arg130, Arg132, Arg236, Arg292, and Arg320 (1,5). The arginines interact with
the nucleotide phosphate oxygens through monodenate and bidenate interactions
(1). These five crucial residues are conserved between creatine kinase
homologues (1,5).
Magnesium ion (Mg2+) is a metal ion
cofactor required for the reaction mechanism. Mg2+ helps align the
substrates of the reaction and stabilize the phosphoryl transfer from ATP to
creatine (1). Mg2+ forms a
complex with ATP when the substrates bind to CKB. It stays in the complex
during the transition state, and an ADP-Mg2+ complex remains even after
the phosphoryl transfer is completed. In the TSAC structure, Mg2+
coordinates with three water molecules, two oxygens from the ADP phosphates,
and an oxygen from a nitrate anion molecule (5). The coordination system
stabilizes the substrates of the reaction.
Creatine has its own binding pocket, separate from the
nucleotide molecule. Ile69 and Val325 form the binding pocket, and Val72 forms
a hydrogen bond with creatine’s main chain nitrogen (1,5). Cys283 also
interacts with creatine and helps to keep the substrate anchored and positioned
for nucleophilic attack on Mg-ATP (1). When creatine binds to CKB, the Cys283
and Glu232 subunits move closer to the phosphate binding pocket (1). Glu232
forms a bidenate interation with the guanidine group on creatine, which turns
creatine into the correct position (5). This is a conformational change—now
different from CKB’s native form—that promotes catalysis (1).
CKB is not the only type of creatine kinase in
the human body. Human brain-type CK and muscle-type CK (CKM) have a high
homology between their primary, secondary, and tertiary structures (1).
PSI-BLAST is used to compare the homology of the primary structure of proteins.
According to a PSI-BLAST of the CKB FASTA sequence, the amino acid sequences
between the brain-type CK and muscle type CK for homo sapiens have an E score
of 0.0. This result suggests that the two homologues are extremely similar. The
PSI-BLAST also showed that CKB and CKM share 81% identities (6). Using Dali to compare the secondary and tertiary
structures between the CKB and CKM homologues produced a Z score of 57.2 (7).
Again, this result demonstrates that the homologues are quite similar in
structure. While these two forms of creatine kinase arise and function in
different tissues, they still retain a high homology. The slight differences
may refer to the differences in location and application. Most differences
between the CKB and CKM forms are seen in the N-terminal region (1). The CKM
N-terminus has an isoenzyme-specific interaction with the sarcomeric M-band and
I-band in muscle tissue (8,1). Greater differences are seen between these
enzymes in their ligand-bound states. CKB exists in a closed form when bound to
ligands (1). By contrast, CKM exists in an open form. Research has narrowed
this difference down to His66 in CKB. Upon substrate binding, His66 (loop
60-70) interacts with Asp326 (loop 323-332), consequently closing the two loops
(1). This conformational change does not occur for CKM.
CKB deficiencies in the brain have been linked to
neurodegenerative diseases, including Alzheimer’s and Huntington’s (HD) (1,9).
As mentioned previously, CKB supplies energy to activate Na+-K+
ATPase ion pumps and ATP-gated K+ channels. Poor regulation of CKB
results in improper action by ion transporters, and thus improper brain
function. In the case of Huntington’s disease, a mutant gene (mHTT) suppresses
CKB (9). This link between CKB and neurodegenerative diseases has lead to
interesting research. Some research suggests that elevating CKB levels or
treating patients with CKB substrate could reduce disease symptoms (9). Further research on CKB could be conducted in order to better understand the mechanism of this pathway, and how it can be manipulated for medical purposes.
 Phosphocreatine is stored in cells and later used as a
rapid source of high-energy phosphate in the form of ATP. The role of CK is
vital to cellular activity. The system functions as an ATP buffer, maintaining
an optimal ATP concentration for cellular activity (2).
Phosphocreatine is stored in cells and later used as a
rapid source of high-energy phosphate in the form of ATP. The role of CK is
vital to cellular activity. The system functions as an ATP buffer, maintaining
an optimal ATP concentration for cellular activity (2).