Myoglobin (PDB ID = 2MM1)
Created by Jiwoong Ahn
alpha helices. Myoglobin is a single-chain globular protein with the heme prosthetic group in the center. four nitrogen atoms of the porphyrin ring, an imidazole side chain of amino acid residue His-64, and an oxygen molecule. Myoglobin is a cytoplasmic hemoprotein, expressed in cardiac myocytes and oxidative skeletal muscle fibers, which are reversibly bound to an oxygen molecule. Myoglobin mainly functions as an oxygen-storage protein in muscle.

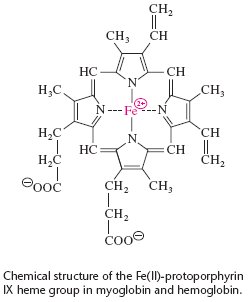
A heme group is the ligand associated with myoglobin where Fe is located at the center of a heterocyclic organic ring, porphyrin. Heme is also a prosthetic group known as a hemoprotein, which exhibits diverse biological functions, including the transportation of diatomic gases, chemical catalysis, diatomic gas detection, and electron transfer. The heme iron also controls electrons during the electron transfer or redox chemistry. The porphyrin molecule can also serve as an electron source in peroxidase reactions. Some diatomic gases can bind to the heme during the transportation. The binding of the oxygen to the heme iron induces conformational changes in the surrounding protein(1).
The iron in the center of the heme group determines the conformation of myoglobin based on its 0.29A towards the planar porphyrin ring(2). If the iron is further oxidized to the ferric state, where the oxidation state is +3, oxygen cannot bind to iron anymore and the myoglobin stays in the covalently bonded to the imidazole ring of His-64. When this histidine complex occupies the iron ligand coordination. His-64 and Val-68 also play significant roles in facilitating reversible oxygen binding and inhibiting further oxidation of Fe to the ferric state. (5)(6).
The assigned myoglobin (pdb id=2mm1) is a recombinant human myoglobin where the lysine at position 45 has been replaced by arginine, and the cysteine at position 110 has been replaced by alanine. Lys-45 in wild-type human myoglobin does not cause iron-ligand coordination. However, Arg- 45 not only causes the iron-ligand coordination, but also changes the overall structure, quite similar to that of the sperm whale myoglobin.