Main players in the Drosophila clock - kinases mentioned in the original text (12)
Drosophila Cryptochrome (PDB ID: 4JZY) from Drosophila melanogaster
Created by: Sina Mazaheri
Drosophila Cryptochrome (dCRY) (PDB ID: 4JZY) , is an FAD-dependent Circadian photoreceptor. It is a member of the Cryptochrome/Photolyase family of photoreceptors. (3, 4) Photolyases are one of the most ancient enzymes, and repair the UV-induced lesions in the DNA by utilizing. UV-light exposure to the DNA can result in the formation of Cyclobutane-Pyrimidine-Dimers (CPD) and 6-4 pyrimidine-pyrimidine photolesions. Photolyases utilize FAD as their cofactor and use blue or near-UV light to revert those lesions back into pyrimidine-pyrimidine dinucleotides (2). From this family of proteins, a specialized class of photoreceptors evolved which are called Cryptochromes. Similar to photolyasase, cryptochromes are activated by blue light, but they have lost their DNA-repairing function. To compensate for that loss, it seems they have acquired C-terminal and N-terminal extensions that have given them a new function, namely signal transduction. Both photolyase and cryptochrome proteins are present in all three kingdoms of life, which hints to their evolutionary importance in the survival of all organisms. (2,8)
Cryptochromes have diverse functions in different organisms. They are regulators of development and growth in plants, function as photoreceptors for the biological clock in some animals including the fruit fly Drosophila Melanogaster , and in higher animals such as mammals they have lost their photoreceptor activity and are essential components of the clock (9). Drosophila Cryptochrome can be found both in the cytoplasm and nucleus, however microscopy results show that the level of cytoplasmic expression is much higher. (Unpublished data). It seems that the expression level of Cryptochrome in the nucleus versus the cytoplasm depends on the species. For example, in Xenopus Cryptochrome has a higher expression in the Nucleus (11).
As mentioned before, in drosophila, Cryptochrome (dCRY) functions as a photoreceptor and helps synchronize the biological clock to the light-dark rhythm in the environment. In response to a pulse of bright light in the subjective night of the animal, fruit flies shift their circadian rhythms to start their activities either earlier or later, depending on the time of the night that the pulse of light is administered. Early light pulses result in a phase-delay (start the next day later), whereas late night pulses of light result in a phase advance (start the next day earlier). dCRY is one of the major proteins that drives these “phase shifts”, which can be looked at as adjustments to the changes in the environmental light-dark rhythms.
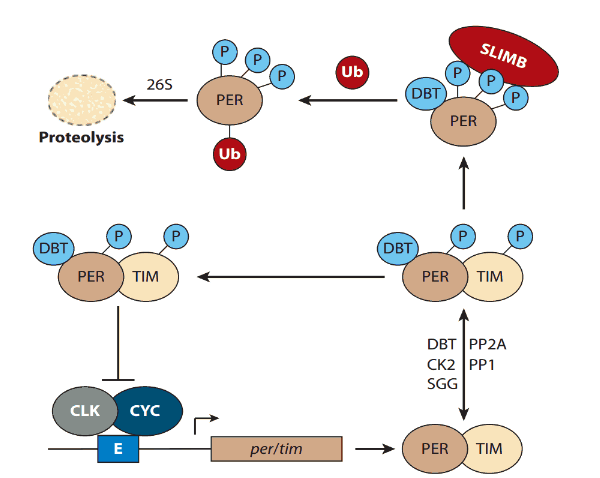
The clock in drosophila consists of a feedback loop, composed of proteins Timeless (TIM) and Period (PER), which suppress their own expression through inhibiting transcription factor dimer Cycle:Clock (CYC:CLK) (9). When exposed to blue light, CRY will change its conformation and bind TIM and target it for proteasomal degradation through an E3 ubiquitin ligase called Jetlag (JET). Cry will be degraded itself by another E3 ligase, called Ramshackle (BRWD3) (3,8,9,10). Also, in the peripheral tissues, dCRY by itself functions as a transcription repressor for CLK and CYC proteins by binding to PER (10).
|
|
|
Main players in the Drosophila clock - kinases mentioned in the original text (12) |
|
|
|
Interaction between dCRY and TIM (13) |
dCRY has an FAD chromophore surrounded by an alpha helical domain. In its ground state, dCRY has FAD in its oxidized state. Reduction of dCRY in response to blue light produces FAD•-, An anionic semiquinone (ASQ). This reduction can also occur through some chemical reducing agents such as dithionite. As a result of this reduction, the C-terminal tail of dCRY, which consists of an alpha-helix, changes its conformation. This changed conformational change results in the signaling state of the protein (9)
The TIM protein has a 10-residue section whose sequence bears a lot of resemblance to the C-terminal tail of dCRY. The sequence is called “C-terminal tail-like” (CTL). It has been shown that a synthetically made sequence similar to the CTL interacts with dCRY when exposed to light (9). This shows that the light-induced conformational change in dCRY C-terminal tail allows for interaction between dCRY and TIM. In other words, the dCRY tail appears to be blocking the interaction between dCRY and TIM in darkness, and CTL of TIM probably binds in the pocket vacated by the conformational change in the dCRY tail (9).
A series of highly conserved Tryptophan residues have been shown to be crucial for the electron transport required for the reduction of FAD in dCRY. These residues are Trp342, Trp397, and Trp420 (4). There are several loops that surround the C-terminal tail, and therefore are worth mentioning. These include 1) the protrusion loop (Phe288 to Ala306); 2) the phosphate binding loop (Glu246 to Met266); 3) the C-terminal lid (Ser426 to Pro440); and 4) the electron-rich sulfur loop, which contains Met331 and Cys337 (4). It seems that the lid, too, goes through some changes that are correlated to the conformational rearrangements in the tail (9).
Another noteworthy sequence of residues arePhe534, Phe535 and Trp536. This FFW triad that is located in the C-terminal tail of dCRY, seems to be important in determining the stability of the protein. Changing these residues to other amino acids reduces the stability of dCRY both in light and dark conditions (9).
dCRY is a monomer made of 536 amino acids, with a molecular weight of 61,799.7 Da and an isoelectric pH of 5.77. It has an extinction coefficient of 108,860 (assuming all Cys residues are reduced). The secondary structure of this protein contains 24 α-helices and 11 β-strands, and 23 turns. The dCRY protein is 47% helical (255 residues) and 7% beta sheet (38 residues) (6). dCRY has two prominent domains; One is the DNA-photolyase domain, that harvests the energy of light, and the other is the FAD-binding site which as the name suggests binds to the cofactor (5).
The dCRY protein was analyzed by two algorithmic protein databases. Using Blast, we checked for proteins with a similar primary structure, while the Dali server helped compare the tertiary structure of dCRY to that of other proteins. Mouse Cryptochrome1 (PDB ID: 4K0R) was found to have a primary sequence 41% identical to the dCRY primary structure (E value=8e-119), and a Z-score of 40.8 (1, 7). This great degree of similarity and retention of tertiary structure hints to the fact that these proteins have optimum shapes and quite significant functions that have enabled the organisms from all three life kingdoms to survive and evolve.