Soybean peroxidase (PDB ID:1FHF) from Glycine max
Created By: J. Singh
Soybean peroxidase (SBP) is an enzyme found in the root, leaf, and hull of the seed Glycine max (Protein Database ID: 1FHF). SBP is an oxidoreductase belonging to class III of the secretory plant peroxidase superfamily. This family of enzymes plays a role in the self-defense system of plants, lignification, and salt tolerance (6). The biological role of soybean peroxidase in particular involves the prevention of premature germination in Glycine max. Mechanistically, SBP catalyzes the oxidation of various organic and inorganic substrates by utilizing hydrogen peroxidase (7).

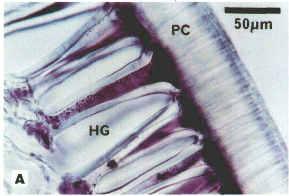
Figure 1. Histochemical Localization of SBP activity in Soybean Seed Coats: HG = Hourglass Cells, PC = Palisade Cells
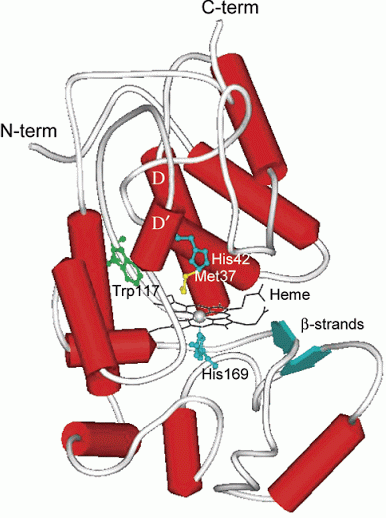
Figure 2. Soybean Peroxidase Schematic from Crystallographic Study (11)
A globular monomeric protein, soybean peroxidase is characterized by three domains according to the SCOP classification system. SBP is a relatively small protein containing 304 amino acid residues and seven glycosylated sites comprising about 18% of the mass of SBP (2, 8). As a member of class III oxidoreductases, SBP is also characterized by thirteen α-helices and two β-strands. The α-helices comprise 45% of the secondary structure of the enzyme, while the β-strands account for 3% (9). A prosthetic group, Fe(III) protoporphyrin IX, as well as four key active site residues help this enzyme fulfill its function. Among these amino acids are His42, Asn70 (hydrogen bonded to His42), Arg38, and Glu64. A second ligand component of SBP is the calcium ion, the function of which remains to be characterized (6). Also bound to the tertiary structure of soybean peroxidase is a molecule of tris(hydroxymethyl)aminomethane (TRIS), which forms a secondary substrate-binding site that remains a topic of future research (7). Together, these three ligands complex with SBP to promote its biological function.
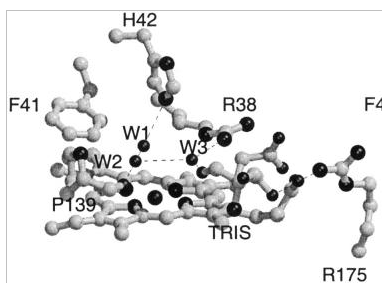
Figure 3. Hydrogen Bonding Pattern in Active Site of SBP (6)
Expert Protein Analysis System (ExPASy) revealed various other physical and chemical parameters of soybean peroxidase (2). This bioinformatics tool provides access to a variety of protein-related analytical tools and databases, integrating this information to a single output. ExPASy search revealed that soybean peroxidase is characterized by a theoretical isolelectric point of 4.61 and a deglycosylated molecular weight of about 33117.1 daltons (2).
Comparisons of enzymatic structures may provide insight into their functional capabilities. For this reason, the Dali server was chosen as a particularly useful resource that allows for comparison of three-dimensional protein structures (12). Horseradish peroxidase C (PDB ID: 1GWU) was identified as the most structurally similar protein to soybean peroxidase, with a Z score of 45.9 indicating high structural alignment. This, along with the following considerations, led to the establishment of HRP C as the best reference molecule for SBP characterization. Both enzymes are categorized as members of the class III peroxidases and share 57% sequence homology. SBP and HRP C share certain key structural characteristics Conserved between these two proteins are the locations of their four characteristic disulfide bridges [slides 12, 13], the presence of a single tryptophan at position 117, and a heme prosthetic group (12). SBP and HRP C also have in common their catalytic mechanism, which begins with a two-electron oxidation of the heme molecule to an intermediate termed Compound I. Subsequent one-electron reductions then ensue to create a second intermediate, followed by a return to the resting state (6):
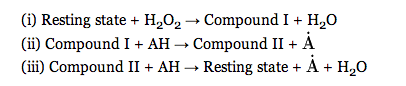
Figure 4. SBP Reaction Mechanism (6)
Although soybean peroxidase shares greatest structural alignment with horseradish peroxidase C, this is not the case for amino acid sequence homology. The BLAST program is designed to compare primary sequence information, with Position-Specific Iterative BLAST (PSI-BLAST) specifically designed to compare protein sequences (1). Soybean peroxidase displays great sequence homology with peroxidase 1 precursor from the species Phaseolus vulgaris. The PSI-BLAST program assigned this homolog an E-value of 2x10-166, indicating almost identical primary structure (1).
Both SBP and HRP C have been proven useful for biosensing and diagnostic applications due to their high stability (11). However, SBP has been shown to possess substantially more thermal stability and catalytic activity, indicating greater potential as a biocatalyst and biosensor (10).
Greater utility of SBP over HRP C may be attributed to various factors. Firstly, soybean peroxidase has been shown to have decreased susceptibility to inactivation by hydrogen peroxidase, resulting in more robust activity (7). Secondly, SBP has high catalytic activity under a wider range of conditions, namely from pH 2 to pH 11 as well as in organic solvents. While HRP C would be inactivated by heme loss at pH 2.4, SBP remains functional to catalyze oxidative reactions (6). Lastly, soybean peroxidase remains active at higher temperatures (Tm = 86°C) (4). SBP has an inactivation temperature of 90.5°C, while that of HRP C is only 81.5 °C. Heat inactivation in both proteins, just as with pH-induced inactivation, also proceeds through the loss of a heme group (6).
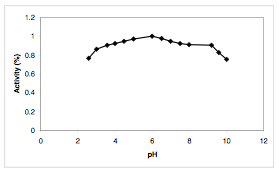
Figure 5. The Effect of pH on SBP Stability (3)
These properties above, along with SBP’s inexpensive and readily available nature in soybean seed coats, enhance its practicality in industrial applications such as wastewater treatment (11). For example, soybean peroxidase holds the potential to oxidize or polymerize hazardous pollutants of petrochemical wastes (6). As a highly stable protein, soybean peroxidase may also hold value in the medical field. Diagnostic toolkits containing this enzyme as a key component have resulted in improved detection of viral, bacterial, and parasitic diseases, including AIDS and malaria (7). SBP was the first enzyme to be used in a peroxidase-based biosensor that was functional at human body temperature of 37°C for an extended period of time. This thus enabled SBP's use in the assessment of various medical conditions, such as diabetes mellitus through the monitoring of glucose and hypoxia and ischemia through monitoring of lactate.
SBP is unique among plant peroxidases in that it contains the most solvent-accessible heme edge (10). This heme group experiences greater exposure under conditions of pH 5.5 due to maximal reduction in the number of β-strands and β-turns. This change is accompanied by observation of maximum catalytic activity and conformational stability of soybean peroxidase (10). Structural comparisons suggest that a significant residue change at position 74 in soybean peroxidase when compared to horseradish peroxidase is likely accountable for the increased heme edge accessibility. The shift from an alanine to bulky isoleucine causes the rotation of the His-42 residue and movement of Phe-41, which together result in the greater exposure of the gamma-meso heme edge. This key structural alteration is anticipated to have importance for the reactivity of SBP, as the the δ-meso edge is believed to be the site of electron transfer from reducing substrates to the enzymatic intermediates, compounds I and II (6).
The increased stability of SBP may also be attributed to a specific crucial amino acid and functional group interactions. For example, the stability of SBP’s heme group may be a product of the contact between the sulfur atom of Met37 and the heme C8 vinyl. The resulting delocalization of the porpphyrin π-cation of compound I in the reaction scheme of soybean peroxidase is hypothesized to affect the stability of the heme group (6).
Soybean peroxidase is widely being acknowledged as a more potent and useful peroxidase than the classical horseradish peroxidase C. The functionality of this enzyme in a wider range of temperature and pH conditions are a direct result of its enhanced stability. While currently a relatively new addition to the scientific literature, soybean peroxidase will surely continue to be the topic of future research as discoveries concerning the root of its stability and catalytic mechanism are unraveled.