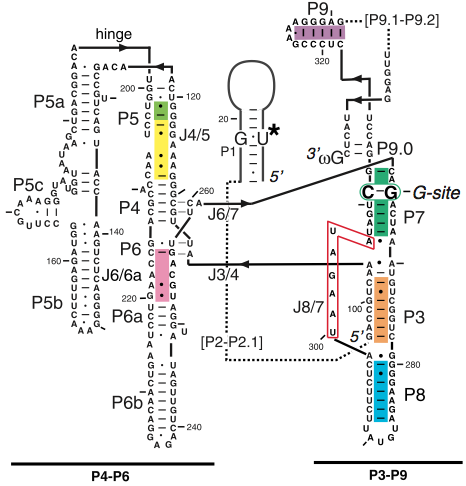

Figure 1. The secondary structure diagram of all three domains that make up the group I intron (4).
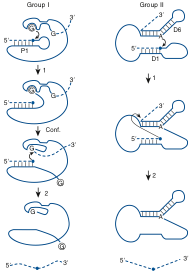
Figure 2. (a) A basic pathway for group I intron self-splicing. Step (1) is the first transesterification that takes place, resulting in a cleavage at the 5’-splice site. The intron goes through a conformation stage (Conf.) where the 3’-guanosine replaces the exogenous guanosine. The splicing comes to completion with the second transesterification step (2) with a cleavage at the 3’-spliesite and ligation of the two exons (5). (b) A basic pathway for group II intron self-splicing. In step (1) an internal adenosine acts as a nucleophile and attacks the 5’-splic site while forming a lariat-type structure. In step (2), the 3’ end of the 5’-exon cleaves the 3’-splice site, ligating the two exons and excising a lariat intron.
|
Residues |
Hydrogen Bond Partner |
|
A-183 |
G-110 |
|
A-184 |
G-212, C-109 |
|
A-186 |
C-137, G-181, G-164 |
|
A-187 |
U-135 |
Table 1. Important hydrogen bonds between the adenosine residues in the A-rich bulge and the nucleotide residues in the P4 segment (6).
|
Residues |
Hydrogen Bond Partner |
|
A-151 |
U-224, A-248 |
|
A-152 |
U-224, C-223, G-250 |
|
A-153 |
C-223, G-250 |
Table 2. Important hydrogen bonds between the adenosine residues in the tetraloop and the nucleotides in the tetraloop receptor region (6).
|
Residues |
|
A-184 |
|
A-186 |
|
A-187 |
|
G-188 |
|
A-171 |
|
A-140 |
|
G-163 |
Table 3. Location of metal ligands in the P4-P6 domain (9).