Alpha-Glycerophosphate Oxidase (PDB ID: 2RGH)
from Streptoccocus Sp.
Created by: J. Murfee
alpha-Glycerophosphate Oxidase from Streptoccocus Sp. (PDB ID= 2RGH) is an oxidizing enzyme found in heme-deficient lactic acid bacteria (2,3,4,8). It is classified as an oxidoreductase and a member of the Flavin Adenine Dinucleotide (FAD)- dependent glycerol-3-phosphate dehydrogenase family, thus leaving GlpO’s to be primarily identified in having an oxidative role in glycerol metabolism as seen in yeasts (5). Specifically, GlpO’s are involved in (FAD)-linked oxidation of α-glycerophosphate to dihydroxyacetone phosphate (DHAP) (2). α-Glycerophosphate oxidase is cytosolic, globular, and dimeric protein whose amino acid sequence is 571 residues long, with a molecular weight of 63,560.1 Da and a theoretical isoelectric point (pI) of 5.19 according to ExPasy (6). alpha-Glycerophosphate's secondary structure displays the layout of its respective beta-sheets, alpha helices, 3-10 heilces, and ranodm coils. GlpO’s have a sequence that is 30-43% identical to sequences of bacterial and mitochondrial α-glycerophosphate dehydrogenases, or GlpD’s (2). Being water soluble, GlpO’s directly catalyze the reduction of O2 à H2O2. This catalysis is different than GlpD’s due to their ability to catalyze the oxidation of Glp à DHAP with accompanying reduction of ubiquinone. This disparity in catalysis due to structural differences is critically important in lactic acid bacteria such as Streptococcus Sp. due to their heme-defecient nature. Heme-deficient organisms lack membrance-associated respiratory chains found in other mitochondria and bacteria (3).
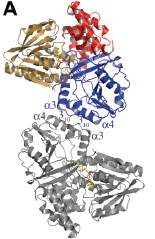
A particular characteristic concerning α-glycerophosphate oxidase’s structure is its 50-residue deletion in its amino acid sequence. These amino acids are Asp356-Ala405 (3). Crytallization of the intact GlpO (that is, the GlpO containing this 50-amino acid insert) and the GlpO molecule without Asp356-Ala405 revealed a common packing interface. This interface is α-glycerophosphate oxidase’s physiologically relevant dimer; both molecules display a similarity in their overall chain-fold (3). Figure 1 (below) illustrates the organization of each chain as three compact domains; Figure 1A denotes domain 1, a FAD-binding domain (colored as sand) consisting of three segments (residues 1-78, 153-254, and 412-452) and a six-stranded beta-sheet. Domain 2 is a dimerization believed substrate-binding domain that consists of two chain segments (residues 59-172 and 255-350) and an eight-stranded beta sheet. Domain 3 is a C-terminal domain is a mostly alpha-helical single strand of the residues 453-571 (3). The results of this crystallization revealed that the 50 amino acid residue absent in the smaller GlpO holds no structural significance; it is merely a segment that bridges the segment of Domain 2 and residues 412-452 of Domain 1.

Figure 1: Figure 1: Image of α-glycerophosphate oxidase’s structure superimposed with another GlpO dimer taken from the article “Structure of α-Glycerophosphate Oxidase from Streptococcus Sp.” (3)
However, proteolysis experiments conducted by Charrier et. al of GlpO expunges residues Ser355-Lys404, which are most of the residues of the 50 amino acid chain. As a result, proteolysis suggests that this 50 amino acid insert represents a dynamic area on the protein’s surface. (2)
With respect to function, α-glycerophosphate oxidase interacts with FAD-proteins to oxidize glycerophosphate, yielding dihydroxyacetone phosphate. Figure 2 (below) diaplays the bent butterfly flavin conformatoin of GlpO (both with and without Asp356-Ala405) with their respective FAD-redox centers:
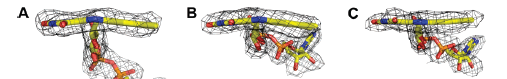
Figure 2:Structures of FAD- redox centers in GlpO missing the 50 amino acid sequence (A), Chain A of the intact GlpO (B), and Chain B of the intact GlpO (C). Taken from “Structure of α-Glycerophosphate Oxidase from Streptococcus Sp.” (3)
The diagrams in Figure 2 illustrate the structural roles that are involved with FAD redox reactions of GlpO’s. Structure A mimics a butterfly conformation for the oxidized FAD in adenosine-5’-phosphosulfate reductase which has a thermodynamically favorable catalysis. Thus, this structural similarity suggests that the bent oxidized flavin form seen in GlpO may have a similar redox influence; GlpO’s effect in supplying a energetically favorable system for Glp dehydrogenation includes an environment that supports the FAD-N1/O2α-anionic form of FADH2 in addition to the butterfly conformation of the oxidized form of FAD (3).
α-Glycerophosphate oxidase’s active site binding occurs near the residues Gly344, Leu345, Arg346, Ile430, and Thr431. On the other side of the substrate-binding pocket, residues His65, Arg 69, Tyr70, and Lys429 provide positively charged effects and hydrogen bonding side chains that interact with the substrate. Due to the nature of the open GlpO structure, the Glp phosphate is attracted to the pocket surrounded by Lys429, His65, Arg69, and Tyr70 with Glp’s C-1 hydroxyl group anchored due to interaction with Arg346 while the Glp substrate approaches the flavin face. The oxidation of the secondary alcohol functional group of Glp requires no acid-base catalyst since the rate of Alanine oxidation is seven times greater in order of magnitude than oxidation of lactate. Interestingly, the GlpO mechanism is distinctive in that it requires an ionization of the C2-OH group of Glp. Ionization mechanisms for NAD+-linked Glp dehydrogenases in both Leishmania Mexicana and the human liver involve a Lys residue in their ionization of the Glp C2-OH; this causes a hydride transfer to the NAD+ acceptor. GlpO ionization cannot mimic these ionization mechanisms due to its His65 residue not being able to abstract the GlpO Cd-OH group due to its hydrogen bonding with Gly67. Thus, the His65 residue of GlpO with the absence of residues Asp356-Ala405 cannot participate in the ionization as an acid-base catalyst unless a conformational change interrupts the interaction (3).
As discussed earlier, α-glycerophosphate oxidase has a sequence that is 30-43% identical to those of mitochondrial and bacterial GlpD’s (2,3,4). A BLAST search, which conducts a comparison of a particular protein’s amino acid sequence to seek any primary structure homologies, yielded glycerol-3-phosphate dehydrogenase (Accession # YP_006374994.1) shared 91% identity with α-glycerophosphate oxidase from Streptococcus Sp. with an E value of 0.0 in regards to their primary amino acid sequences (1). A DALI search, which conducts similarities between proteins based on tertiary structure, indicated aerobic glycerol-3-phosphate dehydrogenase from Escherichia Coli (2r4e-A) to have a 31% homology with α-glycerophosphate oxidase with a Z score of 41.0 (7). Through these results, the conclusion can be reached that difference between GlpO and GlpD amino acid sequence lies with the absence (or, present in the GlpD) of the 50 amino acid sequence Asp356-Ala405. However, with a Dali Z Score of 47.6, this difference serves as only a minor difference in tertiary structure. When comparing the superimposed structure alpha-glycerophosphate oxidase and glycerol-3-phosphate dehydrogenase are very similar.