Activin A
Owais Naeem
Activin A (human; PDB ID 2ARV) is a hormone/growth factor originally thought to be a reproductive hormone. Following its discovery, many additional activities of the protein were described, and it was found in a variety of cell types at all stages of cell and human growth. Some of these functions include stimulating follicle- stimulating hormone (a hormone that causes ovulation), follicle development, binding with follistatin, regulating spermatogenesis, luteolysis, ß-cell proliferation, differentiation into different cell types, and homeostasis. (8).
Activin A is a homodimer with two fully- processed beta-proforms called betaA. Chain A and Chain B both contain 116 amino acid residues. Mature residues usually weigh about 14 kDa in size. The initial strand, containing the precursor and a signal peptide is 426 amino acids long, and contains 13 cysteine residues. (6) The mature subunit is cleaved from its precursor, and only 9 cysteine residues remain. The monomer consists of two antiparallel pairs of ß-strands and a separate a-helix (6). Eight cysteines form four disulfide bonds that form a ring structure in the monomer. The dimer is stabilized by the ninth cysteine, creating a disulfide bond with another monomer. The subunits of the dimer are positioned in a way that the alpha- helix of one subunit interacts through hydrophobic contact with the ß-sheet surface of the second subunit. (6) Research through mutagenesis on the cysteines to see their biological function has showed that cysteine 4 and 12 are not necessary for dimerization to occur. Cysteine 80 is the cysteine that forms a disulfide bond to create a dimer, and without this amino acid, a 14 kDa monomer forms. These monomers have only 2% biological activity of activin. This suggests that the dimerization is necessary for biological activity. (6) A non-covalent stabilizing action occurs between the cys-80 and the valine-82, and that may be the reason why biological activity is so hindered when the cysteine has mutated to alanine. Therefore, if high affinity receptor binding is to occur, a dimer must form (6)
Fibroblasts, hepatocytes, neurons, macrophages, Leydig cells, and Sertoli cells, are all cells known to express betaAchains. Activin is produced in the gonads, pituitary gland, and placenta. In the ovarian follicle, activin increases follicle stimulating hormone (FSH) binding. It also enhances luteinizing hormone action in the ovary and testis. In males, activin enhances spermatogenesis. Activin is strongly expressed in wounded skin, and research on transgenic mice shows improved healing and scar formation. When activin is missing in the body during growth, some developmental defects may also form.
The entire sequence of activin A is conserved in greater then 200 species or queries done by BLAST. If a sequence is highly conserved, this means that the protein is of vital importance to the organism, and remains consistent throughout evolution. Furthermore, a conserved domain search shows that the genetic sequence of Activin only contains domains found in the TGF-ß superfamily.
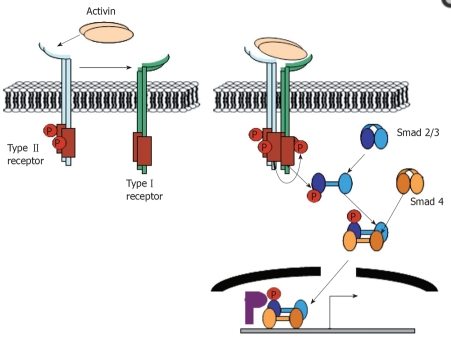
TGF-ß’s are small signaling proteins that regulate cell growth and differentiation. They belong to the larger superfamily along with activin and bone morphogenetic proteins (BMP). TGF- ß undergo a variety of functions. In mammals, TGF- ß molecules control sexual development, pituitary hormone production, and production of bones and cartilage. (54) Activin A goes through a complex of transmembrane receptor kinases. It binds to Activin A receptors type II (ActRIIA or ActRIIB) and then recruits Activin A receptor type IB (ALK-4). ALK-4 interacts with and phosphorylates SMAD2 and SMAD3. SMAD4 binds to phosphorylated SMAD2 and SMAD3 and this complex translocates to the nucleus. After translocation into the nucleus, SMAD2 and SMAD3 may activate transcription of different genes. (1)

Fig. 1 diagram of activin receptors and SMAD proteins (2)
The activin receptors are transmembrane serine/threonine kinases similar to other members of the TGF-beta superfamily. There is a type II ligand-binding receptor, a type I signal-transducing receptor, and possibly a type III ligand-presenting receptor.
Follistatin and Follistatin-like 3 (FSRP) bind to Activin A with a high affinity, and can inhibit it from binding to the receptors. During bone formation, an interaction between FS and activin A can be involved in the development and conversion of cartilage to bone
Activin and inhibin are two very closely related proteins that have opposite biological effects. While activin enhances follicle- stimulating hormone secretion, inhibin down regulates its synthesis and inhibits its secretion. Activin is a dimer composed of two beta subunits. Activin A is a homodimer composed of two similar betaAsubunits. Inhibin is also a dimer, but it is composed of one beta subunit and a distantly- related alpha subunit. (3) A disulfide bond links the two chains. The alpha and beta subunits share approximately 25% sequence similarity, whereas the similarity between beta subunits is approximately 65% (5)
Knock-out mice for the inhibin α subunit developed gonadal sex-cord stromal tumors suggesting a tumor suppressive function of the inhibin α subunit. (3) In both males and females, inhibin inhibits FSH production and Gonadotropal releasing hormone (GnRH) release from the hypothalamus.
In contrast to activin, less is known about the mechanism of inhibin. It may involve competing with activin for binding to activin receptors and/or binding to inhibin-specific receptors. (3)
Activin A exerts its biological function and binds to types of serine/ threonine kinase receptors, type I and type II receptors. The high affinity binding of these receptors is necessary for signal transduction. (7)
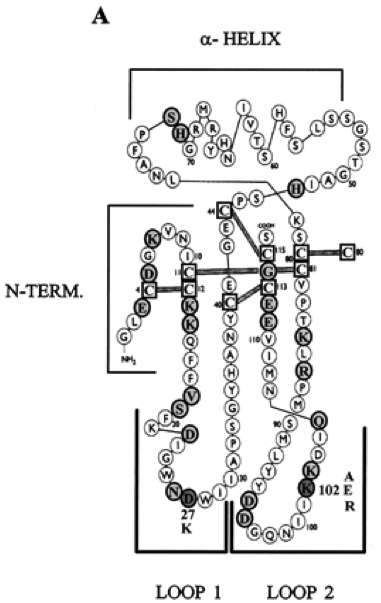
Figure 2. Schematic diagram of the Activin monomer (7)
In Figure 2, residues that did not significantly alter wild-type activity are highlighted in light grey, and mutations that caused affinity to increase or decrease compared to the wild-type is highlighted in dark grey. Mutational analysis showed that two amino acids, K102 in loop 2, and D27 in loop 1, are important for biological activity based on their ability to stimulate FSH release by gonadotropic pituitary cells. Postiviely charged K102 displayed the activin function, while D27 produced lower biological function then its mutation. (7) Activin’s structure is a key to ensuring that the binding occurs with high affinity so biological processes such as follicle development, sex determination, testicular function, glucose metabolism, and immune response can all occur. (8) This protein is highly important in human and cell growth, and new functions of this protein will continue to be found as more research is done. It is in fact a protein essential for life, and this can be observed through its conservation through evolutionary history, and its relation to human growth.